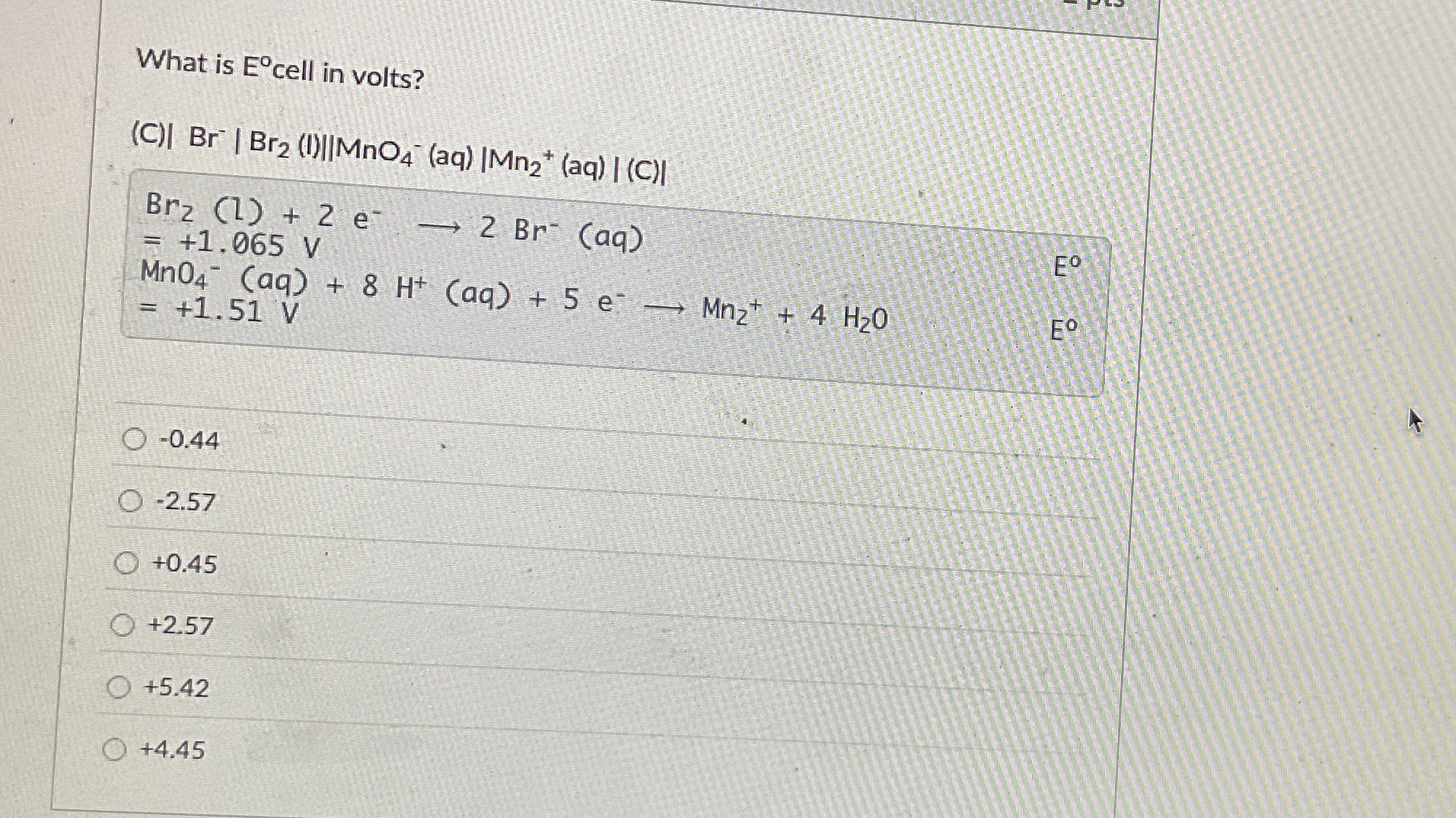Home /
Expert Answers /
Chemistry /
what-is-e-deg-cell-in-volts-c-br-br-2-i-mno-4-aq-mn-2-aq-c-br-pa205
(Solved): What is E\deg cell in volts? (C) |Br^(-)|Br_(2)(I)||MnO_(4)^(-)(aq)|Mn_(2)^(+)|(aq) ||(C) || Br_( ...
What is
E\deg cell in volts? (C)
|Br^(-)|Br_(2)(I)||MnO_(4)^(-)(aq)|Mn_(2)^(+)|(aq)
||(C)
||
Br_(2)(l)+2e^(-)->2Br^(-)(aq)
=+1.065V^(-)
MnO_(4)^(-)(aq)+8H^(+)(aq)+5e^(-)longrightarrowMn_(2)^(+)+4H_(2)O
=+1.51V
-0.44
-2.57
+0.45
+2.57
+5.42
+4.45