Home /
Expert Answers /
Mechanical Engineering /
the-compression-ratio-of-an-otto-cycle-as-shown-in-figure-22-13-is-va-vb-8-00-at-the-beginn-pa229
(Solved): The compression ratio of an Otto cycle as shown in Figure 22.13 is VA/VB=8.00. At the beginn ...
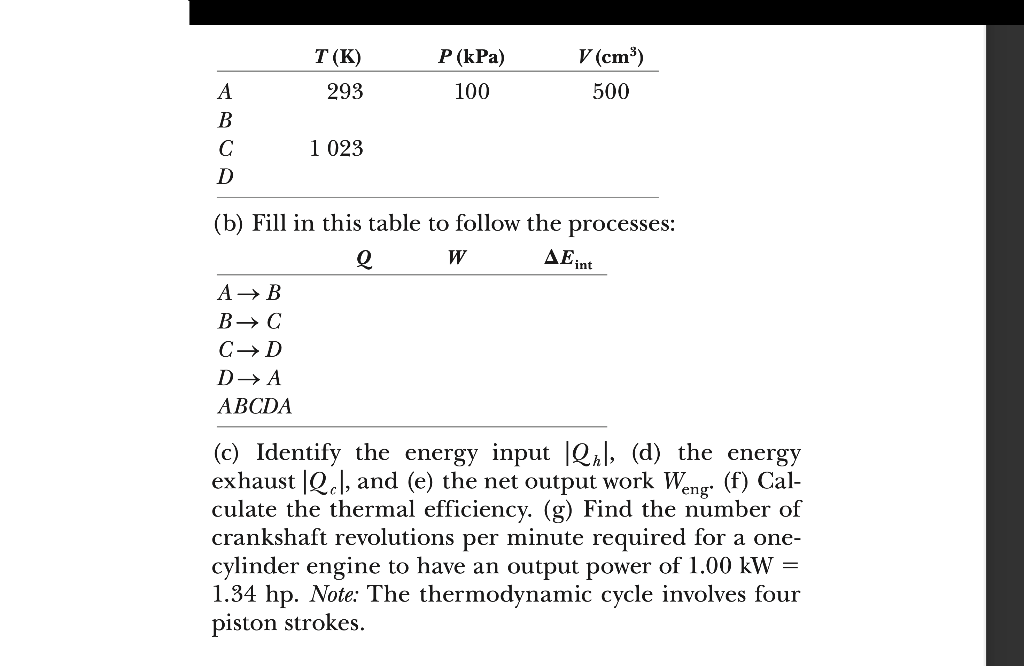
The compression ratio of an Otto cycle as shown in Figure 22.13 is . At the beginning of the compression process, of gas is at and . At the beginning of the adiabatic expansion, the temperature is . Model the working fluid as an ideal gas with . (a) Fill in this table to follow the states of the gas:
(b) Fill in this table to follow the processes: (c) Identify the energy input , (d) the energy exhaust , and (e) the net output work (f) Calculate the thermal efficiency. (g) Find the number of crankshaft revolutions per minute required for a onecylinder engine to have an output power of . Note: The thermodynamic cycle involves four piston strokes.
Expert Answer
(a) The complete table is shown belowGiven Info: The compression ratio of an Otto cycle is VA/VB = 8, at the beginning of A, the initial volume of the gas is 500 cm3 and the initial temperature is 20 degree C and 100kPa. At the beginning of the adiabatic expansion, the temperature is TC = 750 degree C. The adiabatic index is 1.4.The expression to calculate the quantity of the gas isHere,PA is the pressure of gas at point AVA is the volume of gas at point ATA is the temperature of gas at point AR is the universal gas constant.Substitute 100kPa for PA , 500cm3 for VA , 20 deg C for TA and 8.314 J/mol . K for R in above equation to find n. = 0.0205 molIn process A B ,Pressure at point B PB = PA Substitute 8 for , 1.40 for and 100 KPa for in the above equationThe compression ratio is, = 8Substitute 500 cm3 for VA in the above equation. The expression to calculate the temperature at point B is Substitute 1.84 x 106 Pa for PB , 62.5cm3 for VB , 0.0205 for n and 8.314 J/mol . K for R in above equation. = 673 KAt state C:VC = VB Pressure at point C.Substitute 62.5cm3 for VC , 0.0205 for n and 8.314 J/mol . K for R and 750 dec C fot TC in above equation. = 2 .79 x 106 Pa = 2 .79 x 103 kPaState D:VD = VA and VC = VB = Pressure at point DPD = PC Substitute 2 .79 x 106 Pa for PC and 1/8 for in the above equationTemperature at D Substitute 1.52 x 105 Pa for PD , 500cm3 for VD , 0.0205 for n and 8.314 J/mol K for R in the above equationFrom the above explanation, the complete table is given below.