Home /
Expert Answers /
Chemistry /
na-nh3-alkynes-are-reduced-to-trans-alkenes-by-a-process-called-dissolving-metal-reduction-pa466
(Solved): Na,NH3 Alkynes are reduced to trans alkenes by a process called dissolving metal reduction ...
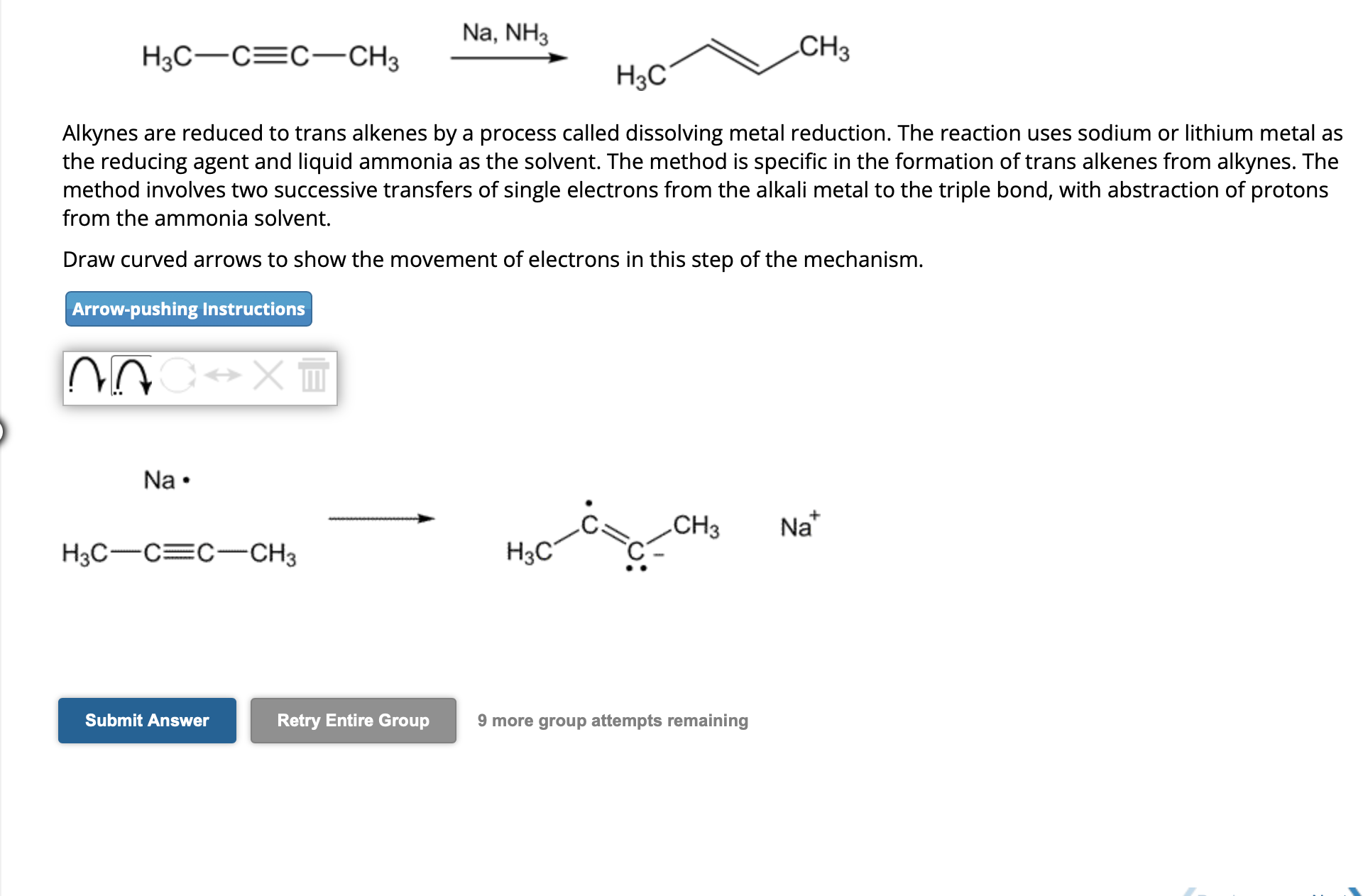
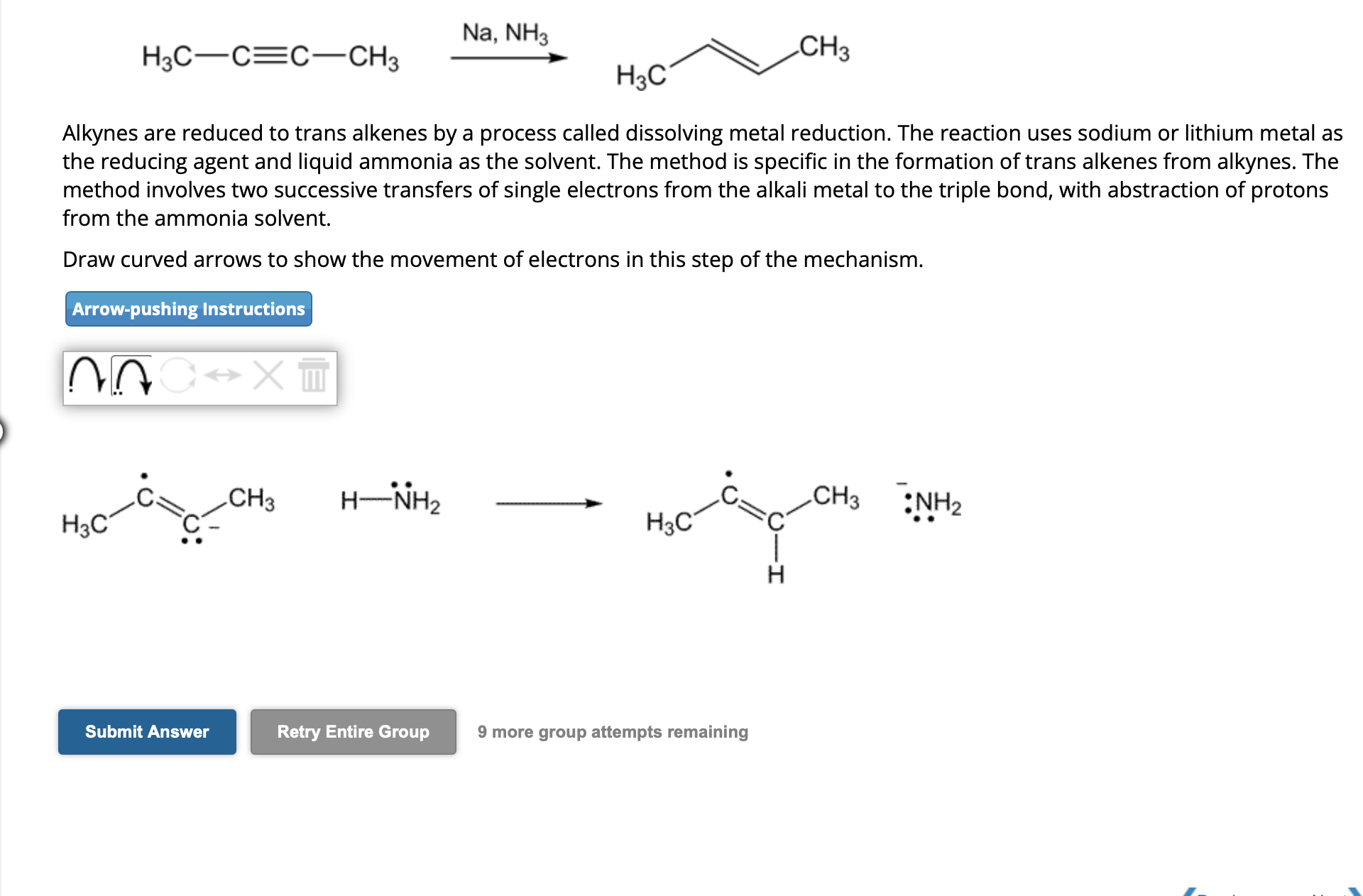
Alkynes are reduced to trans alkenes by a process called dissolving metal reduction. The reaction uses sodium or lithium metal as the reducing agent and liquid ammonia as the solvent. The method is specific in the formation of trans alkenes from alkynes. The method involves two successive transfers of single electrons from the alkali metal to the triple bond, with abstraction of protons from the ammonia solvent. Draw curved arrows to show the movement of electrons in this step of the mechanism. 9 more group attempts remaining
Alkynes are reduced to trans alkenes by a process called dissolving metal reduction. The reaction uses sodium or lithium metal as the reducing agent and liquid ammonia as the solvent. The method is specific in the formation of trans alkenes from alkynes. The method involves two successive transfers of single electrons from the alkali metal to the triple bond, with abstraction of protons from the ammonia solvent. Draw curved arrows to show the movement of electrons in this step of the mechanism.
Expert Answer
The question is related to the topic of reducing regents which is most important part of organic chemistry Let us understand the reduction of Alkynes in presence sodium in liquid ammonia (Na,NH3) which are provided below