Home /
Expert Answers /
Chemistry /
draw-the-lewis-structure-of-each-species-and-determine-the-number-of-bonding-and-lone-pairs-of-ele-pa204
(Solved): Draw the Lewis structure of each species and determine the number of bonding and lone pairs of ele ...
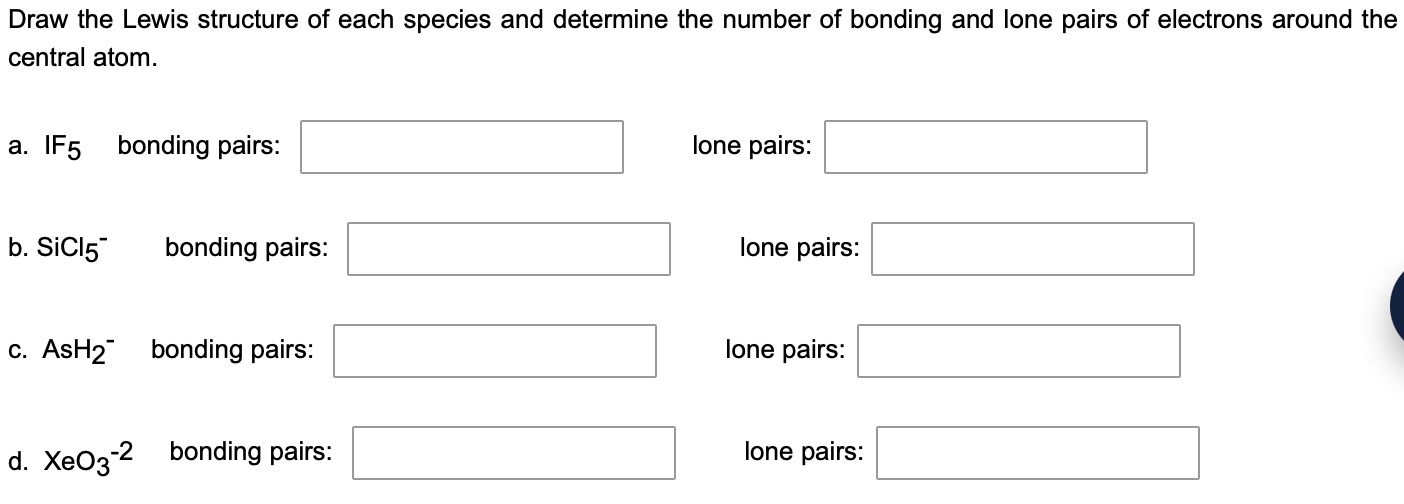
Draw the Lewis structure of each species and determine the number of bonding and lone pairs of electrons around the central atom. a. bonding pairs: lone pairs: b. bonding pairs: lone pairs: c. bonding pairs: lone pairs: d. bonding pairs: lone pairs:
Expert Answer
Part a)The Molecule is The central atom is The atomic number of is and valence electrons is The number of bond pairs is ( five sigma bonds)The number of lone pairs is The total number of electron pairs is Hybridization is Electron pair geometry is Molecular geometry is The Lewis structure is shown below.For six electron domains the electron pair geometry is octahedral when there is one lone pair the shape changes to square pyramidal to minimize bond-pair lone-pair repulsion