Home /
Expert Answers /
Chemistry /
classify-each-of-the-following-as-a-strong-acid-or-a-weak-acid-indicate-how-each-should-be-written-pa248
(Solved): Classify each of the following as a strong acid or a weak acid. Indicate how each should be written ...
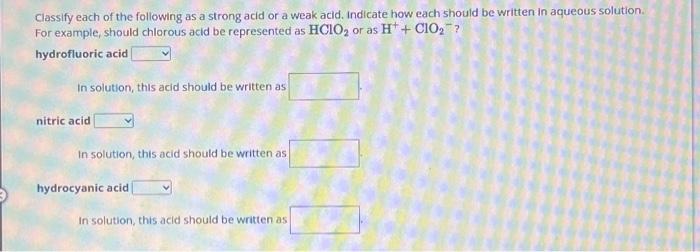
Classify each of the following as a strong acid or a weak acid. Indicate how each should be written in aqueous solution. For example, should chlorous acid be represented as or as ? hydrofluoric acid In solution, this acid should be written as nitric acid In solution, this acid should be written as hydrocyanic acid In solution, this acid should be written as
Expert Answer
The following compounds in aqueous solution are written as below:1) Hydrofluoric acid (HF)