Home /
Expert Answers /
Chemistry /
a-what-volume-of-1-00-m-naoh-is-required-to-neutralize-the-following-solution-26-3-ml-of-0-152-m-pa288
(Solved): a. What volume of 1.00 M NaOH is required to neutralize the following solution? 26.3 mL of 0.152 M ...
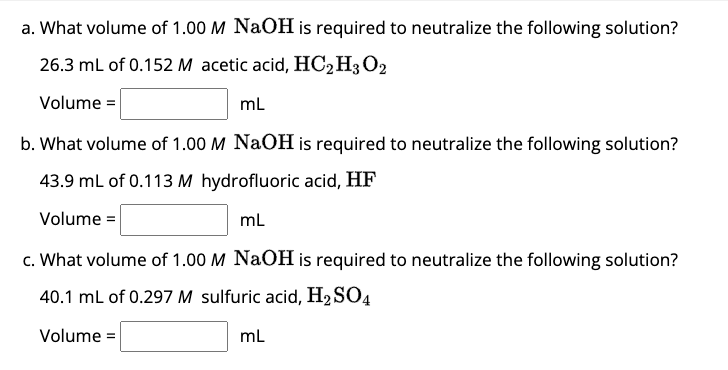
a. What volume of 1.00 M NaOH is required to neutralize the following solution? 26.3 mL of 0.152 M acetic acid,
HC_(2)H_(3)O_(2)Volume
=mL b. What volume of 1.00 M NaOH is required to neutralize the following solution? 43.9 mL of 0.113 M hydrofluoric acid, HF Volume
=mL c. What volume of 1.00 M NaOH is required to neutralize the following solution? 40.1 mL of 0.297 M sulfuric acid,
H_(2)SO_(4)Volume
=mL